From mycopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
MnP는 다양한 phenol 과 dye 를 산화 시킬수 있다 . | MnP는 다양한 phenol 과 dye 를 산화 시킬수 있다 . | ||
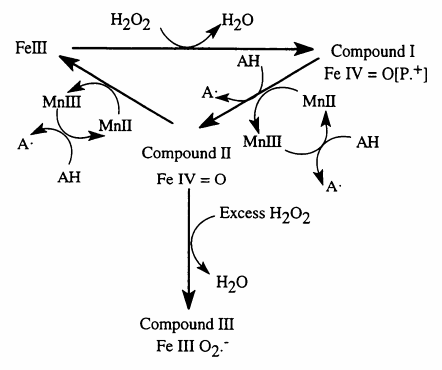
| + | *Catalytic cycle of manganese peroxidase | ||
[[File:Manganese peroxidase.GIF ]] | [[File:Manganese peroxidase.GIF ]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
*fungal taxa : Basidiomycota | *fungal taxa : Basidiomycota | ||
Revision as of 16:26, 29 June 2011
Enzyme number :EC 1.11.1.13
H2O2 에 의존 하여 Mn2+ 를 Mn3+ 로 산화 시키고 , Mn3+ 가 유기 물질을 산화 시키게 된다 . iron protoporphyrin IX group 을 가지고 있는 glycoprotein 이다 .
MnP는 다양한 phenol 과 dye 를 산화 시킬수 있다 .
- Catalytic cycle of manganese peroxidase
- fungal taxa : Basidiomycota
- Localization : Extracellular
• H2O2-dependent one-electron oxidation of Mn2+ to Mn3+, which subsequently oxidizes organic compounds • Redox potential of 1.0–1.2 V • Mn3+-mediated oxidation of various phenols and aromatic amines • Extended substrate range in the presence of co-oxidants (organic SH-containing compounds, unsaturated fatty acids and their derivatives) • Activity in the acidic pH range 60,68,70