From mycopedia
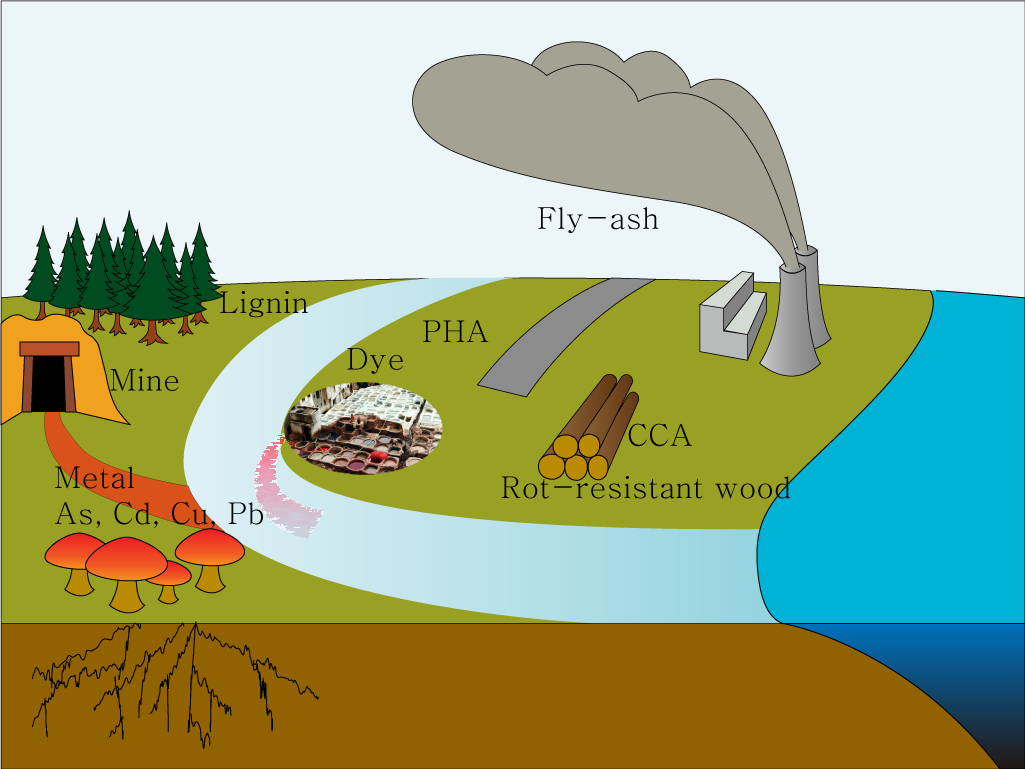
Mycoremediation is a process of using fungi to degrade or detoxify contaminants in the environment.mycoremediation is actively investigated in these days as a potent bioremediation tool. For example, brown rot fungi can absorb heavy metals by chelating with oxalic acids while white rot fungi can degrade phenolic compounds (e.g dyes) from wastewater. Some fungi have diverse abilities to degrage or modify environmental pollutants. The low specificity of fungal enzymes and their independence from using pollutants as a growth substrate make these fungi well suited for bioremeidation processes.
- Materials for Fungal Biodegradation and Biodeterioration
| Wood | Plastics | Library materials |
| Wooden artifacts | Wool | Wall paintings |
| Stored paper | Wrapping papers | Electroinsulating materials |
| Textiles | Glass surfaces | Coal |
| Leather | Concrete | Ground waste rubber materials |
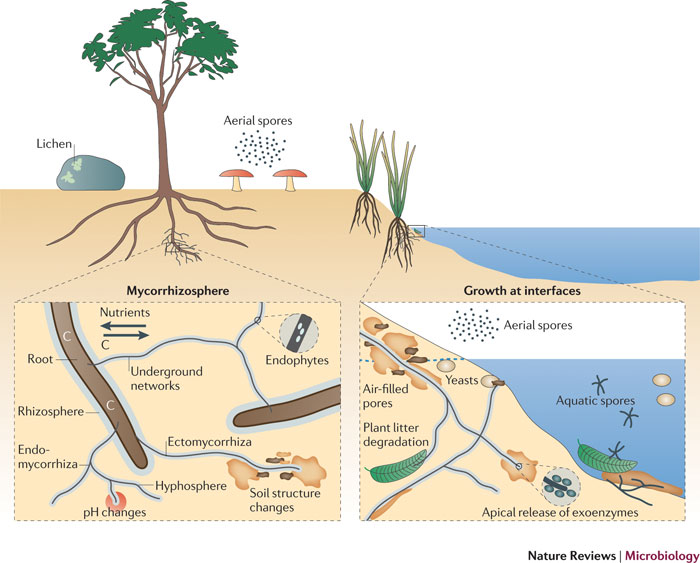
Fungi in the environment can exist as terrestrial and aquatic saprobes, yeasts, symbiotic partners of lichens, and mycorrhizal symbionts associated with plant roots. They can form ectomycorrhizal and endomycorrhizal associations and can influence soil structure (by enmeshment of particles), soil chemistry (by excretion of acids, for example) and plant growth (through the mobilization and provision of nutrients in exchange for photosynthetic assimilates). Saprotrophic fungi adapted to water-unsaturated soil or aqueous environments can propagate through spores adapted to dispersal through the atmosphere or water, respectively. Growing hyphae explore food sources by deploying extracellular enzymes (exoenzymes).
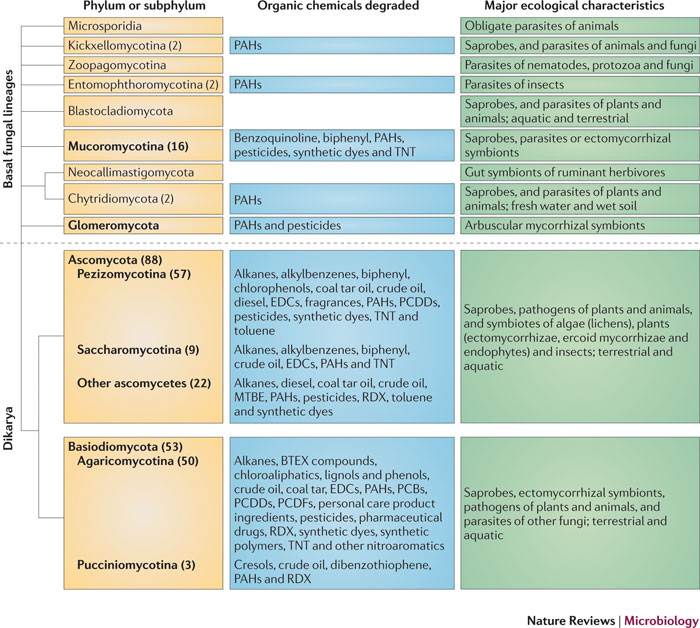
Ligninolytic fungi
PAHs , benzene/toluene/ethyl benzene/xylene (BTEX) ,synthetic substituted aromatics
KUC fungi
Laccases (EC 1.10.3.2)
Fungal taxa : Ascomycota and Basidiomycota
Localization and occurrence: Extracellular
Reaction mechanism: O2-dependent one-electron oxidation of organic compounds
- • Redox potential of around 0.4–0.8 V
- • Direct oxidation of various phenols, aromatic amines and anthraquinone dyes
- • A wide range of pollutants oxidized in the presence of natural and synthetic redox mediators
- • Activity mostly in the acidic and rarely in the neutral or alkaline pH range
Tyrosinases (EC 1.14.18.1)
Fungal taxa : Ascomycota, Basidiomycota and Mucoromycotina
Localization and occurrence: Sometimes extracellular but mainly intracellular
Reaction mechanism:
- • O2-dependent hydroxylation of monophenols to o-diphenols (cresolase activity)
- • Oxidation of o-diphenols to catechols (catecholase activity)
- • Oxidation of various phenols, including those that are highly chlorinated
- • Activity from the acidic to the alkaline pH range
Lignin peroxidases (EC 1.11.1.14)
Fungal taxa : Basidiomycota
Localization and occurrence: Extracellular
Reaction mechanism:
- • H2O2-dependent one-electron oxidation of aromatic compounds
- • Redox potential of 1.4–1.5 V
- • Direct oxidation of various aromatics with high redox potentials, but rapid inactivation during oxidation of phenols
- • Direct oxidation of PAHs with an ionization potential of ≤7.55 eV
- • Extended substrate range (including dyes with high redox potentials and phenols) in the presence of the redox mediator veratryl alcohol
- • Activity in the acidic pH range
Manganese peroxidases (EC 1.11.1.13)
Fungal taxa : Basidiomycota
Localization and occurrence: Extracellular
Reaction mechanism:
- • H2O2-dependent one-electron oxidation of Mn2+ to Mn3+, which subsequently oxidizes organic compounds
- • Redox potential of 1.0–1.2 V
- • Mn3+-mediated oxidation of various phenols and aromatic amines
- • Extended substrate range in the presence of co-oxidants (organic SH-containing compounds, unsaturated fatty acids and their derivatives)
- • Activity in the acidic pH range
Versatile peroxidases (EC 1.11.1.16)
Fungal taxa :Basidiomycota
Localization and occurrence:Extracellular
Reaction mechanism:
- • H2O2-dependent direct one-electron oxidation of aromatic compounds
- • H2O2-dependent one-electron oxidation of Mn2+ to Mn3+, which subsequently oxidizes organic compounds
- • Redox potential of around 1.4–1.5 V
- • Direct oxidation of phenols and aromatics with high redox potentials, including dyes
- • Mn3+-dependent reactions as for manganese peroxidase
- • Activity in the acidic pH range
Coprinopsis cinerea peroxidase (EC 1.11.1.7)
Fungal taxa :Basidiomycota
Localization and occurrence:Extracellular
Reaction mechanism:
- • H2O2-dependent one-electron oxidation of aromatic compounds
- • Redox potential of around 0.9–1.1 V
- • Direct oxidation of phenols and dyes with low redox potentials
- • Activity from the acidic to the alkaline pH range
Dye-decolorizing peroxidases (EC 1.11.1.x)
Fungal taxa :Basidiomycota
Localization and occurrence: Extracellular
Reaction mechanism:
- • H2O2-dependent one-electron oxidation of organic compounds
- • Additional hydrolysing activity
- • Redox potential of around 1.2–1.5 V
- • Oxidation of anthraquinone dyes with high redox potentials (only rarely oxidized by other peroxidases)
- • Highly stable at high pressure, high temperature and very low pH
- • Activity in the acidic pH range
Caldariomyces fumago haem–thiolate chloroperoxidase (EC 1.11.1.10)
Fungal taxa :Ascomycota
Localization and occurrence:Extracellular
Reaction mechanism:
- • H2O2-dependent halogenation of organic compounds in the presence of halides (one-electron transfer)
- • H2O2-dependent one-electron oxidations of phenols and anilines in the absence of halides
- • H2O2-dependent peroxygenation (two-electron oxidation), leading to epoxidation of (cyclo)alkenes, hydroxylation of benzylic carbon and sulphoxidation of S-containing organic compounds
- • Redox potential not known
- • No activity on non-substituted aromatic rings and n-alkanes
- • Activity in the acidic pH range
Haem–thiolate peroxygenases
Fungal taxa : Basidiomycota
Localization and occurrence: Extracellular
Reaction mechanism:
- • H2O2-dependent peroxygenation of aromatic, aliphatic and heterocyclic compounds, leading to aromatic and alkylic carbon hydroxylation, double-bond epoxidation, ether cleavage, sulphoxidation or N-oxidation reactions (depending on the substrate);
- • H2O2-dependent one-electron abstractions from phenols;
- • H2O2-dependent bromination of organic substrates
- • Redox potential not known
- • Peroxygenation of various monoaromatic to polyaromatic pollutants, including PAHs, dibenzofuran, and monohydroxylated and polyhydroxylated products
- • Ether bond cleavage between aromatic and aliphatic parts of molecules and in alicyclic and aliphatic ethers (for example, MTBE)
- • Activity from the acidic to the alkaline pH range
Cytochrome P450 monooxygenases
Fungal taxa : Ascomycota, Basidiomycota, Mucoromycotina and Chytridiomycota
Localization and occurrence: Cell bound
Reaction mechanism:
- • Incorporation of a single atom from O2 into a substrate molecule, with concomitant reduction of the other atom to H2O
- • Epoxidation and hydroxylation of aromatic or aliphatic structures of many pollutants, including PAHs, PCDDs, alkanes and alkyl-substituted aromatics
Phenol 2-monooxygenases (EC 1.14.13.7)
Fungal taxa : Ascomycota and Basidiomycota
Localization and occurrence: Cell bound
Reaction mechanism:
- • Incorporation of a single atom from O2 into a substrate molecule, with concomitant reduction of the other atom to H2O
- • Ortho-hydroxlyation of various (halo)phenols to the corresponding catechols
Nitroreductases
Fungal taxa : Ascomycota and Basidiomycota and Mucoromycotina
Localization and occurrence: Cell bound
Reaction mechanism:
- • NAD(P)H-dependent reduction of nitroaromatics to hydroxylamino and amino(nitro) compounds, and of nitro functional groups of N-containing heterocycles
- • Reduction of TNT to hydroxylamino-dinitrotoluene and amino-dinitrotoluenes
- • Formation of mononitroso derivatives and ring cleavage products from cyclic nitramine explosives
- • Widespread among fungi
Quinone reductases
Fungal taxa :Basidiomycota
Localization and occurrence: Cell bound
Reaction mechanism:
- • NAD(P)H-dependent reduction of quinones
- • Functions in quinone detoxification, in the conversion of quinones arising from extracellular pollutant oxidation into substrates for extracellular and intracellular oxidoreductases, and in pollutant attack by hydroxyl radicals arising from quinone redox cycling
- • Occurrence in white-rot and brown-rot basidiomycetes
Reductive dehalogenases
Fungal taxa : Basidiomycota and perhaps Ascomycota
Localization and occurrence: Cell bound
Reaction mechanism:
- • Two-component system comprising a membrane-bound glutathione S-transferase that produces glutathionyl conjugates with concomitant chlorine removal, and a soluble glutathione conjugate reductase that releases reductively dechlorinated compounds
- • Reductive dechlorination of chlorohydroquinones arising from chlorophenol degradation and of diphenyl ether herbicides (basiodiomycetes)
- • Perhaps responsible for reductive dechlorination of chlorocatechols arising from PCDD degradation (ascomycetes)
Miscellaneous transferases
Fungal taxa : Ascomycota, Basidiomycota and Mucoromycotina
Localization and occurrence:Cell bound
Reaction mechanism:
- • Formation of glucoside, glucuronide, xyloside, sulphate or methyl conjugates from hydroxylated compounds
- • Phase II enzymes are prominent in fungal PAH metabolism but also act on other pollutants
- • Widespread among fungi
Untapped potential: exploiting fungi in bioremediation of hazardous chemicals Hauke Harms, Dietmar Schlosser & Lukas Y. Wick Nature Reviews Microbiology 9, 177-192 (March 2011) doi:10.1038/nrmicro2519